The Philadelphia chromosome, a genetic anomaly first identified in the 1960s, remains a pivotal discovery in the field of cancer research. This specific chromosomal abnormality is primarily associated with chronic myelogenous leukemia (CML), a type of blood cancer. Understanding its origins and implications has led to groundbreaking advances in treatment options and a deeper understanding of how certain cancers develop. Despite the vast strides made in the field, the exact mechanism by which the Philadelphia chromosome contributes to cancer remains a subject of ongoing research. This blog will delve into the mystery of the Philadelphia chromosome, exploring its discovery, its role in cancer, and how it has shaped modern medicine.
The Discovery of the Philadelphia Chromosome
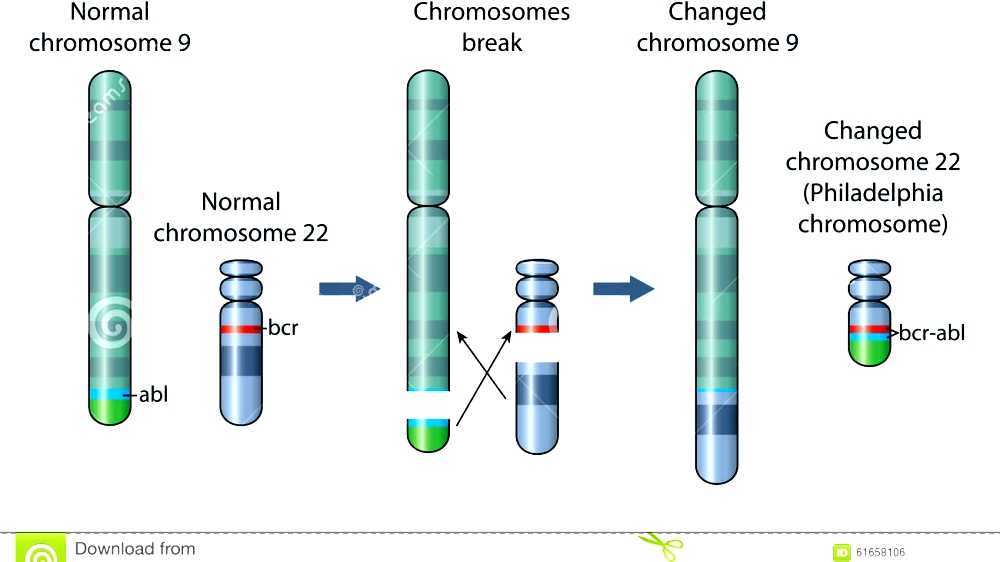
The Philadelphia chromosome was first discovered by researchers Peter Nowell and David Hungerford in 1960. They identified a shortened version of chromosome 22 in leukemia patients, a finding that would eventually lead to a deeper understanding of the disease. This discovery revolutionized the way scientists viewed genetic mutations in cancer, particularly in hematologic malignancies. The abnormal chromosome, later named the Philadelphia chromosome, was linked to chromosomal translocations, where genetic material from one chromosome is transferred to another. This discovery laid the groundwork for understanding the molecular mechanisms behind many cancers, particularly blood cancers like CML.
How the Philadelphia Chromosome Causes Cancer
The Philadelphia chromosome results from a specific translocation between chromosome 9 and chromosome 22. This translocation leads to the formation of a fusion gene called BCR-ABL1, which encodes an abnormal protein. This protein has tyrosine kinase activity, which leads to uncontrolled cell division, a hallmark of cancer. The BCR-ABL1 fusion protein interferes with normal cell signaling pathways, causing the cells to grow and divide uncontrollably. This uncontrolled division results in the accumulation of leukemia cells in the bone marrow and blood, which ultimately leads to cancer.
The Role of BCR-ABL1 in Leukemia
The BCR-ABL1 fusion gene is a key player in the development of chronic myelogenous leukemia (CML) and some other cancers. This fusion gene produces the BCR-ABL1 protein, which is constantly active, driving the uncontrolled growth of white blood cells. This leads to the buildup of abnormal cells, which crowd out healthy blood cells and impair normal blood function. The presence of the BCR-ABL1 gene is a diagnostic marker for CML and has been crucial for targeted therapy. The discovery of this gene paved the way for the development of treatments like imatinib, which specifically targets the BCR-ABL1 protein.
Targeted Therapy: A Breakthrough in CML Treatment
Targeted therapy, which involves drugs that specifically target the BCR-ABL1 protein, has been a game-changer for CML patients. Imatinib, the first and most well-known of these drugs, has been shown to dramatically improve survival rates for those with Philadelphia chromosome-positive CML. These targeted therapies work by inhibiting the activity of the BCR-ABL1 protein, thereby halting the uncontrolled cell division that causes leukemia. This treatment has led to remission in many patients and has revolutionized cancer treatment by offering a more precise and less toxic alternative to traditional chemotherapy. The advent of targeted therapies based on the Philadelphia chromosome represents a major milestone in personalized medicine.
Philadelphia Chromosome and Other Cancers
While the Philadelphia chromosome is most closely linked to chronic myelogenous leukemia, its presence has also been identified in other cancers, such as acute lymphoblastic leukemia (ALL). In these cases, the BCR-ABL1 fusion gene can be found, though it is less common than in CML. The fusion gene can alter the development of other cancers by disrupting normal cell function. Understanding its role in various cancers has broadened the scope of molecular cancer research and improved diagnosis and treatment strategies. Ongoing research continues to explore the role of the Philadelphia chromosome in other malignancies, which could open up new avenues for targeted therapies.
Vote
Who is your all-time favorite president?
Advances in Diagnostic Techniques
The identification of the Philadelphia chromosome has greatly advanced the field of cancer diagnostics. Advances in genetic testing, such as fluorescence in situ hybridization (FISH) and polymerase chain reaction (PCR), have made it easier to detect the BCR-ABL1 fusion gene. These tests are crucial in diagnosing CML and determining the specific type of leukemia a patient may have. Early detection of the Philadelphia chromosome allows for early intervention, which can significantly improve patient outcomes. The ability to detect the presence of the Philadelphia chromosome has also contributed to the development of personalized treatment regimens based on the specific genetic makeup of the cancer.
The Role of the Philadelphia Chromosome in Cancer Resistance
While treatments like imatinib have been revolutionary, the emergence of resistance to these therapies poses a significant challenge. Some patients with the Philadelphia chromosome-positive CML develop mutations in the BCR-ABL1 gene, which makes the fusion protein less susceptible to treatment. These mutations can lead to treatment failure, requiring alternative therapies or the development of second-generation tyrosine kinase inhibitors. Understanding the mutations associated with resistance is a key focus of ongoing research aimed at improving the effectiveness of treatment. This is a reminder that even with targeted therapies, cancer treatment remains a dynamic and evolving challenge.
Ongoing Research and Clinical Trials
Research into the Philadelphia chromosome is ongoing, with numerous clinical trials exploring new treatment options and better diagnostic tools. These trials aim to improve outcomes for patients who either do not respond to current treatments or who experience relapses. Researchers are also investigating the use of combination therapies, which could include tyrosine kinase inhibitors in conjunction with other treatments like immunotherapy or chemotherapy. The goal is to develop treatments that are even more effective, less toxic, and tailored to each patient’s genetic profile. Emerging therapies could potentially offer new hope for those affected by cancers related to the Philadelphia chromosome.
The Broader Implications for Genetics and Cancer
The discovery of the Philadelphia chromosome has had a profound impact on the broader field of cancer genetics. It was one of the first examples of a genetic mutation being directly linked to a specific cancer, which has paved the way for similar discoveries in other cancers. This discovery highlighted the importance of genetics in cancer development and treatment, leading to the rise of precision medicine. It also underscored the need for ongoing research into the molecular mechanisms behind cancer. As scientists continue to study the Philadelphia chromosome, we may uncover even more genetic insights that could change the future of cancer care.
Key Contributions of the Philadelphia Chromosome to Cancer Research
- Identification of the BCR-ABL1 fusion gene – A key step in understanding CML.
- Advancements in targeted therapies – Leading to the development of drugs like imatinib.
- Improved cancer diagnostics – Through genetic testing like FISH and PCR.
- Personalized medicine – Tailoring treatments based on genetic profiles.
- New treatment possibilities – Investigating second-generation inhibitors for drug resistance.
- Broader cancer insights – Understanding the role of genetics in various cancers.
- Ongoing research – Continually evolving our understanding of genetic mutations in cancer.
Watch Live Sports Now!
Dont miss a single moment of your favorite sports. Tune in to live matches, exclusive coverage, and expert analysis.
Start watching top-tier sports action now!
Watch NowThe Impact of Philadelphia Chromosome in CML Treatment
- Life-saving therapies – Targeted drugs that reduce CML symptoms.
- Improvements in remission rates – Significant advancements in survival.
- Reduced treatment toxicity – More precise therapies with fewer side effects.
- Faster diagnosis – Genetic tests allow for earlier detection.
- Personalized treatment plans – Tailoring therapies to the individual patient’s genetic profile.
- Continued resistance studies – Ongoing research into overcoming treatment resistance.
- Hope for the future – Paving the way for potential breakthroughs in other cancers.
Pro Tip: If you or a loved one is diagnosed with Philadelphia chromosome-positive CML, ensure regular genetic testing to monitor the progression and effectiveness of treatments.
| Trait | Impact | Clinical Relevance |
|---|---|---|
| BCR-ABL1 fusion gene | Leads to uncontrolled cell growth | Key in diagnosing CML and other cancers |
| Tyrosine kinase activity | Drives cancer cell division | Targeted therapy inhibits this activity |
| Resistance mutations | Reduces treatment efficacy | Need for alternative therapies |
“The discovery of the Philadelphia chromosome was a breakthrough in cancer research, opening doors for better diagnosis and personalized treatment.”
Understanding the Philadelphia chromosome has led to immense progress in cancer treatment, offering new hope to many patients. The breakthroughs in diagnostics and therapies have saved countless lives and continue to shape the future of personalized medicine. Whether you are a patient, researcher, or healthcare professional, staying informed about the latest findings can help drive progress in the fight against cancer. Share this article with friends and family to spread awareness and encourage further research into genetic-driven treatments. Bookmark this post to stay up-to-date on the ongoing advancements in cancer care, and let’s continue to explore the mysteries of genetics together!